neutron
-

The Proton-Proton Chain
Take a wild guess: how much energy do you think the sun generates? Think about it. It definitely generates enough energy to power a world. Humans depend on the photosynthesis of plants, which converts sunlight into energy. And that’s not…
-
The Battery of the Sun
Does this image look familiar? It should — these are soap bubbles. Okay, now you’re probably going to ask me how soap bubbles have anything to do with the battery of the sun. Well…you might be surprised to know that…
-

How Atoms Work
Have you ever seen something like this? I’m going to venture a wild guess and say you haven’t, since scientists have only recently been able to take this kind of image. I learned about it in my biology class this…
-
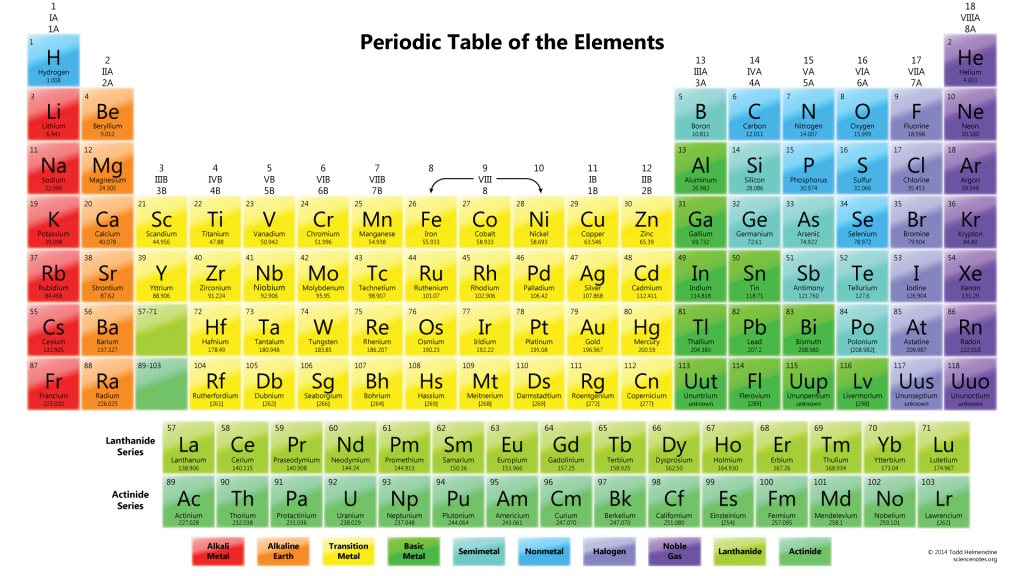
Types of Atoms
Does this look familiar? It might, or it might not. If it does, you might recognize it as the periodic table of the elements — more often known as simply the “periodic table.” It’s an ingenious way to organize elements…
-

The Building Blocks of the Universe
“The Building Blocks of the Universe.” When you put it that way, atoms sound less like a topic specifically for a chemistry class and more like something astronomers might discuss. They really are. I’ve got a fantastic reason to include…



