Welcome to my fourth “Science Answers” post! If you have a question, you can ask it in the comments here, or ask it in an email. Or find me on Facebook!
Q: (1) How did scientists find elements in the first place? Could there be more undiscovered elements?
(2) How did scientists create the periodic table?
(3) How do we know that everything is made up of atoms, when atoms are so small that they can’t even reflect light (a necessity for seeing them)?
(asked by Mukesh Garbyal)
Really good questions! I was asked these in a comment on my post “Types of Atoms,” and chose to answer them in a post of their own.
Let’s take this apart. I actually want to address the third part of the question first, since it contains a misconception: atoms can reflect light. Their interaction with light is actually why we can see anything in the world.
How?
As I believe most of us were taught in school, atoms are made up of three particles: protons, neutrons, and electrons.
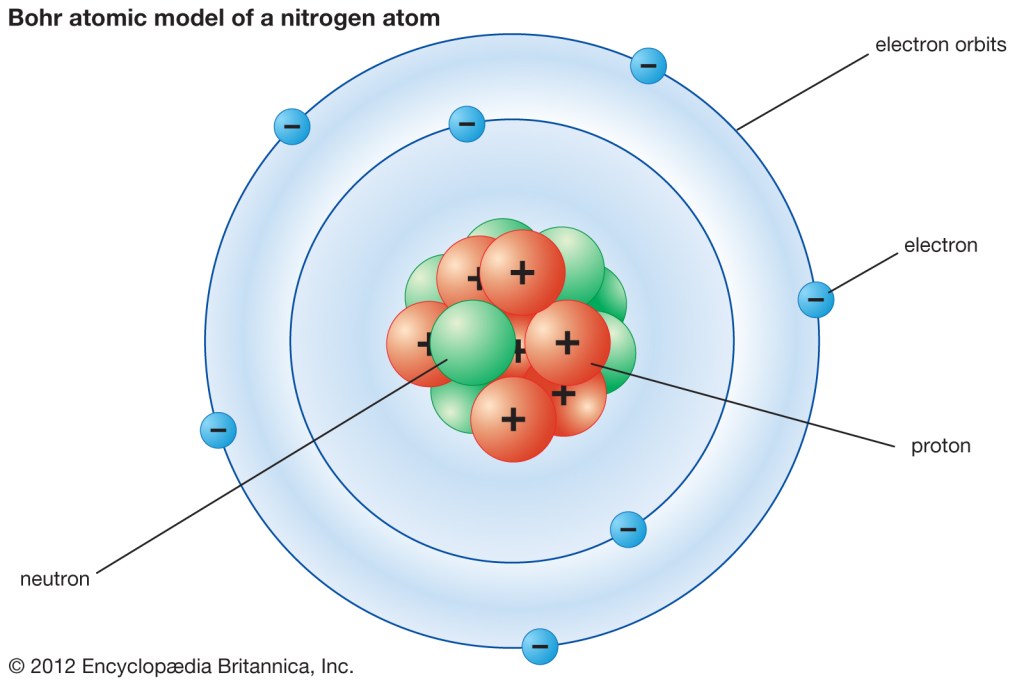
Right now, let’s completely ignore the nucleus, that cluster of protons and neutrons in the center. We only care about the electrons, because they will interact with light.
We also care about how much energy each of these electrons has. Each one has a specific amount of energy.
Now let’s take a look at what exactly light is—this will be important for understanding how it interacts with atoms.

This is the full spectrum of light that exists in the universe (that we know of). Notice that only a very small portion is visible to our eyes—that’s the colors of the rainbow. The rest, however, is just as important.
Notice, also, that the different kinds of light also have different amounts of energy. In fact, each different wavelength of light has a specific amount of energy.
Sound familiar? So do our electrons.
What happens if light hits an electron?
First, let’s take another look at electron energies.

Electrons can only have specific amounts of energy, like rungs on a ladder. Just as you can’t hover midair in the space between rungs, electrons can’t have energies that are between certain energy levels.
Think of energy levels as different sized orbits around the nucleus. An electron needs more energy to orbit farther from the nucleus than to orbit closer to the nucleus.
This means that if light hits an electron, it has to have the right amount of of energy. If it’s too much or too little, the electron won’t be able to absorb it.
But what if it is the right amount of energy?
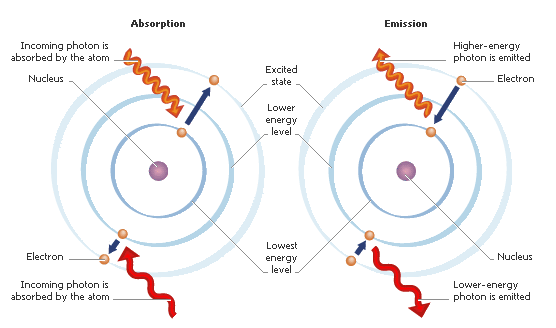
When the right amount of energy from a photon—essentially a particle—of light hits an electron, it absorbs that energy and moves up to the next energy level of the atom. It may even skip a level or two, depending on how much energy it absorbed.
But here’s the important part. The electron won’t stay in the higher energy level forever. Soon, it will drop back down to its ground state, and it will release the same amount of energy it absorbed.
This is what we mean when we say that light reflects off of matter (and atoms are the smallest unit of matter). Light actually gets absorbed and re-radiated by the electrons inside the matter.
So what does this mean for the world we see around us?
Well, there’s two kinds of colors: light and pigment.

Light is, well, radiation. The electromagnetic spectrum. It’s the stuff that interacts with electrons.
Pigment is paint, ink, etc. It’s the stuff that colors the world around us. It’s the paints you played with in kindergarten.
Pigments are made up of atoms, so they contain electrons. Light interacts with the electrons in the pigments, and photons of certain energies are re-radiated to reach our eyes.
That’s how we see anything in the world.
Now for the next question: How did scientists find elements in the first place? Could there be more undiscovered elements?
The short answer is: yes, there could be.
The long answer starts with a man named John Dalton, who lived from 1766-1844. Interestingly enough, he was actually an English schoolteacher. (That is, he was English—that’s not the subject he taught.)

Dalton is credited as being the first person to perform experiments that tested and corrected his ideas about atoms. There are very likely other people who co-discovered atoms, though.
Dalton built on the work of Antoine Lavoisier and Joseph Proust, who had studied compounds called tin oxides. These are molecular compounds that occur in specific ratios of tin to oxygen.

Proust had found that the masses of tin oxides are either 88.1% tin and 11.9% oxygen or 78.7% tin and 21.3% oxygen. Dalton noticed that 100g of tin will combine with either 13.5g or 27g of oxygen, and that these numbers form a ratio of 1:2.
It didn’t stop there—Dalton tested other compounds, and found that they all combine in ratios. An elegant way to explain this was to assume that the basis of these elements—tin and oxygen—is a single atom.
The thing is, half an atom can’t combine with another atom. There isn’t even such thing as half an atom, because splitting an atom just gives you a different, smaller atom.
This simple building block which cannot be broken down further—the atom—is the simplest way to explain why elements always combine in very even ratios.
Countless experiments since have supported the idea that the elements of the periodic table are made up of basic building blocks called atoms. And so, over time, scientists have accepted the atom as a well-tested theory—which, among scientists, is something that might as well be a fact.
To answer the second part of that question—could there be more undiscovered elements—I’m going to first answer the asker’s second question: How did scientists create the periodic table?
First, let’s take a look at this periodic table we keep talking about.

The periodic table is a chart that organizes all elements according to properties—characteristics—they have in common. In order to answer our questions, let’s sift through the bounty of information the periodic table offers.
First, you’ll notice that the elements are numbered left to right, top to bottom. Hydrogen is #1; #118, down at the bottom right, is Oganesson. But what do those numbers mean?
This is the element’s atomic number. It refers to how many protons are in an atom.
Wait…what are protons again?

Protons are the positively charged particles that make up the central nucleus of an atom.
Earlier, we dismissed protons, but now, they’re very important to us because they make the difference between atoms.
Yeah. Seriously. Add a proton to an atom, and you have an entirely new (and slightly heavier) atom. Subtract one, and you have a slightly lighter atom.
So, if different elements are defined by their number of protons, and there are no blank spots on the periodic table…doesn’t that seem to indicate we’ve discovered them all?
Nope. We could always discover atoms heavier than Oganesson. But these are most often synthesized in laboratories, not found naturally.
But…how was the periodic table created in the first place?
Like I said, elements are organized according to their atomic number (their number of protons) and a number of different properties.

I chose this periodic table to display because it clearly labels the different types of elements. Notice that it’s color-coded according to the key up at the top?
All elements on the periodic table are either alkali metals, alkaline-earth metals, other metals, noble gases, rare-earth elements and lanthanoid elements, actinoid elements, or other nonmetals. Notice how all elements of a certain type are grouped together?
Okay…I do see that there are a few exceptions. For example, why isn’t hydrogen (H) grouped with the other alkaline-earth metals? And why aren’t all the lanthanides (yellow) and actinides (blue) grouped together?
That’s because there are a few other properties the periodic table organizes elements by—namely, the specific way their electrons orbit the nucleus.

Notice that hydrogen has only a single electron in the red-colored energy level—known in more advanced chemistry as “1n.” Lithium (Li) and sodium (Na) also have only one electron in their 1n energy level.
Lithium and sodium are alkali metals, not alkaline-earth metals, but sorting by electron orbits wins out.
The same goes for the lanthanide and actinide series at the bottom of the table. You’ll notice that no matter what periodic table you look at, they are always lined up under the same columns—known as groups—of the main body of the periodic table.
In addition to grouping elements by alkali, alkaline, noble gases, etc, rows—known as periods (hence the periodic table)—sort elements by which energy levels are occupied by electrons, as you see in the chart above.
To sum up…
- Atoms are perfectly capable of reflecting light, in fact their reradiating of light is why we can see anything.
- Atoms were discovered by examining their ratios in chemical compounds, and more are always being discovered in laboratories.
- The periodic table is organized by element properties, such as the number of protons, the type of element, and the way the element’s electrons orbit the nucleus.
Hope that answers your questions, Mukesh! Thanks so much for asking!