matter
-

The Big Bang in a Nutshell
Okay, okay, I know…with that featured image, I just broke my own cardinal rule of not depicting the Big Bang as an explosion. I did pick that image for a reason, though. We first covered the Big Bang waaaay back…
-

The Problem of Matter
At first glance, this is an ordinary image of a galaxy cluster: specifically, Abell 1689. If we look closer, though, we see that there’s something weird going on with the more distant, background galaxies in the image. Many of those…
-

Star Mass and Density
What makes a star shine bright? Much earlier on — probably months ago now — I explained how something called the proton-proton chain generates massive amounts of energy within stars, and enables them to fuel whole solar systems. That’s the battery…
-

The Proton-Proton Chain
Take a wild guess: how much energy do you think the sun generates? Think about it. It definitely generates enough energy to power a world. Humans depend on the photosynthesis of plants, which converts sunlight into energy. And that’s not…
-
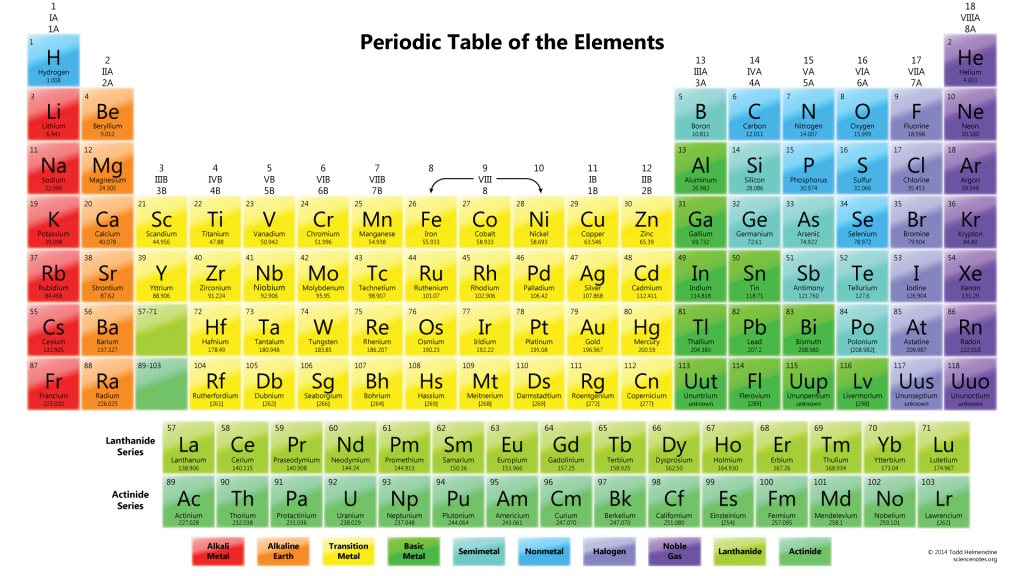
The Building Blocks of the Universe
“The Building Blocks of the Universe.” When you put it that way, atoms sound less like a topic specifically for a chemistry class and more like something astronomers might discuss. They really are. I’ve got a fantastic reason to include…
-

Newton’s Laws of Motion
It’s said that Sir Isaac Newton was sitting under an apple tree when an apple fell on his head, and that’s when all his discoveries began. Personally, I doubt that story — just as I doubt that Galileo Galilei ever dropped…
-

What Matters?
The simplest approach to chemistry is to start basic. Not basic as in acids and bases, ha-ha…sorry, bad chemistry joke. I mean basic as in, what the heck even is chemistry? I admit that I’m better versed in astronomy than…


